By Sammy Caiola
The Sacramento Bee.
SACRAMENTO
Depression lifted from Nick O’Madden’s life like a set of foggy glasses being wiped clean.
Earlier this summer, O’Madden, 31, felt he was living in a distracted haze, sprinkled with nighttime panic attacks. Now, after undergoing an emerging high-tech treatment involving magnetic currents, he said he’s literally seeing the world in a new light.
“Colors are brighter,” said O’Madden, a mental health therapist who lives in Elk Grove, Calif. “Last night, I was looking at the moon, and it just looked clearer and brighter and more beautiful … It’s almost kind of scary to see that at first, it’s so new to me.”
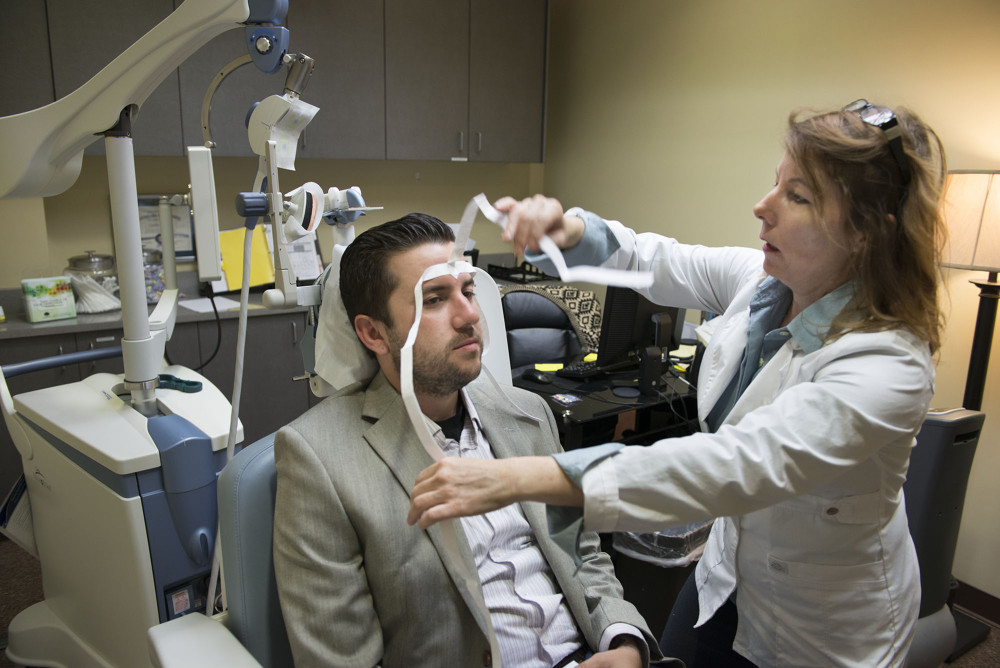
He described the changes from a reclined medical chair at TMS Health Solutions, a treatment center in Sacramento’s Campus Commons area that specializes in transcranial magnetic stimulation. With a metal coil positioned near his forehead, he spoke between bouts of jackhammer-esque pulsing that erupted every 15 seconds during the 50-minute session.
The “train pulses,” as technicians call the strings of sound, are actually the back-and-forth flexing of the metal coil as the device sends out a 2-tesla-strong magnetic current. The coil creates a magnetic field that reaches 2 to 3 centimeters into brain matter to stimulate the dorsal-lateral prefrontal cortex, the poker chip-sized area responsible for regulating mood, memory and decision-making.
An estimated 7 percent of American adults suffer from clinical depression, which can cause lethargy, indifference, moodiness and other symptoms that interfere with day-to-day functioning. Researchers have found that the prefrontal cortex is often underactive in people diagnosed with the illness.
The TMS technology uses electric currents to excite cell activity in that part of the brain, theoretically helping neurons better communicate with one another and increasing blood flow to the tissue, which promotes healthy brain function. The federal Food and Drug Administration has approved the treatment, but for limited use given that it is relatively new.
Physicians can administer it only to patients who haven’t responded to at least one prescription medication for depression.
Advocates of the treatment refer to it as revolutionary; there hasn’t been a major development in procedure-based depression treatment since the advent of electroconvulsive therapy. Unlike that treatment, which applies electricity directly to the skull to invoke a brain seizure, the magnetic TMS procedure has not shown negative impact on memory or cognition.
TMS, and other new treatments that focus on physical stimulation of specific parts of the brain, signal a departure from traditional methods of treating mental illness, such as talk therapy and prescription drugs. As advances in medical technology allow researchers to better understand how the human brain functions, approaches to treatment are following suit.
“There’s a growing recognition, both with scientists and patients, that depression is a brain problem, a problem with chemicals in our circuitry,” said Dr. Paul Croarkin, a psychiatrist with the Mayo Clinic. “The fact that we’re soon going to have more and more offerings in that regard is a positive thing.”
As with many emerging medical treatments, practitioners and insurers hesitate to embrace new procedures without a solid track record. Though the FDA approved the nation’s first TMS device in 2008, major health insurance companies have only begun covering the treatment in the past few years.
Dr. Richard Bermudes is principal owner and medical director of TMS Health Solutions and president of the nationwide Clinical TMS Society. By his estimate, about 700 TMS devices are in use in the United States.
The treatment has been the focus of about 30 randomized clinical trials in the United States, and in most of those, a statistically significant portion of patients were determined to have benefited. But some researchers caution that the improvements could be the result of a placebo effect, and have called for longer-term studies.
A 2012 study published in the Journal of Clinical Psychiatry showed that half of the patients involved in that trial responded to the treatment within six weeks, and 25 percent went into remission. Still, guidelines from the Agency for Healthcare Research and Quality and the Department of Health and Human Services state that evidence is “insufficient to evaluate the ability of (repetitive) TMS to maintain response or remission.”
On the upside, the therapy has not been shown to cause whole-body side effects, such as weight gain or fatigue, as an oral medication might, said Dr. Guohua Xia, a clinical associate professor at University of California-Davis and medical director of Brainefit, a mental health institute offering a form of TMS services. That means it can be especially helpful for pregnant women and elderly people who may experience problems with standard depression drugs.
Sutter Health is the only hospital system in Sacramento to offer the treatment. Dr. Theodore Goodman, director of interventional psychiatry for Sutter Health, said TMS has resulted in improvement for about 60 percent of patients during the two years it has been offered at the Sutter Center for Psychiatry.
He expressed frustration at insurance companies not covering the treatment long term. TMS is currently approved only as a treatment for acute clinical depression, meaning patients are in the throes of symptoms.
“About 50 percent of patients will relapse over a year’s time,” he said. “The way you avoid that is maintenance treatment, but insurance won’t pay for that. It’s a horribly unenlightened viewpoint.”
While NeuroStar, the model used at Bermudes’ clinic, was the first TMS device approved on the market, other brands go even further into brain matter and have been used to target other conditions, including migraines.
At TMS Health Solutions, the recommended treatment plan is four to six weeks of daily TMS sessions, at a cost of $5,000 to $7,000. Since opening clinics in El Dorado Hills, Calif., in 2007 and Sacramento in 2010, Bermudes said, he has used the technology to treat hundreds of patients and currently sees about 20 TMS patients a day.
He said he recently changed the clinic’s name, from Mindful Health Solutions, specifically to highlight the treatment.
“There’s not a lot of access to this modality currently in the community,” he said. “A lot of our patients have been depressed for years before coming to TMS. If we can get people into TMS earlier, our remission and response rates may go up.”
O’Madden said that, for him, improvement came after three weeks of treatment, though technicians say results can take longer. Before his physician suggested TMS, O’Madden said, he was having trouble sleeping and focusing, and struggled to control anxiety and obsessive thought patterns.
He said he’s now feeling more focused and active, with a renewed passion for boxing. He recently started a blog to document his recovery.
“I want people to become more aware of (TMS), and I also want mental health to be less stigmatized,” he said. “I want people to see me as a person: I’m a father and a husband, but I suffer from a mental health disorder, and I’m seeking help … and other people can do the same thing.”