Kim Christensen
Los Angeles Times
WWR Article Summary (tl;dr) As Kim Christensen reports, “The surgery left [Wendy] Knecht, now 65, with nerve damage and in chronic pain. It also led to a malpractice lawsuit, two complaints to the Medical Board of California and a push for legislation requiring doctors to tell patients if they have accepted payments from the makers of drugs and medical devices.”
Los Angeles
Wendy Knecht figures she’s been failed twice by doctors — once by the plastic surgeon she accused of botching her breast reconstruction, and again by the California physicians lobby fighting a proposed law that would force doctors to alert patients to potential conflicts of interests.
Knecht, a Studio City writer and health care advocate, was diagnosed in December 2014 with the BRCA2 gene mutation, which has been linked to an increased risk of breast and ovarian cancers.
The former Pan Am flight attendant, whose mother died of breast cancer, decided to reduce her risk by having a preventive double mastectomy.
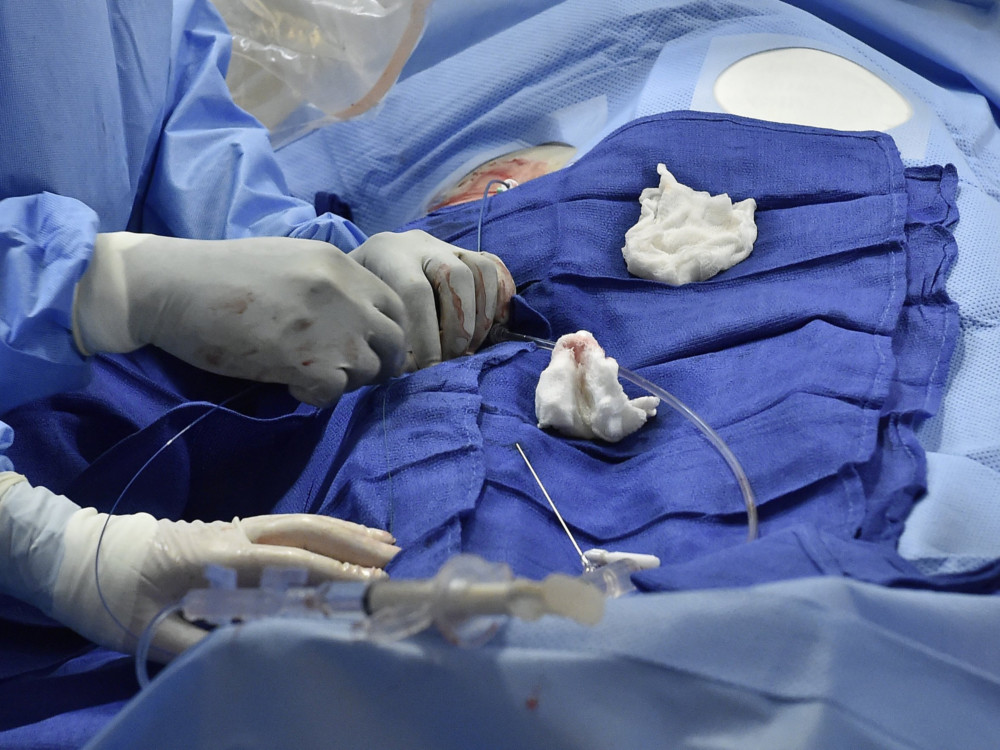
The surgery left Knecht, now 65, with nerve damage and in chronic pain. It also led to a malpractice lawsuit, two complaints to the Medical Board of California and a push for legislation requiring doctors to tell patients if they have accepted payments from the makers of drugs and medical devices.
AB 1278 is working its way through the Legislature but was amended amid strong opposition from the California Medical Assn. so that it no longer requires doctors to inform patients about such payments.
“It’s really disheartening,” Knecht said. “When a physicians group is against patient care and transparency and informed consent, that’s upsetting.”
Anthony York, spokesman for the CMA, said the group is all for transparency but considered the disclosure requirements too burdensome. The association has said the disclosures would bury doctors in paperwork and could disrupt vital patient care.
“Disclosure itself is something CMA supports,” York said. “The concern we have is trying to protect the sanctity of the patient-physician visit.”
Knecht’s ordeal began six years ago, when she had a bilateral mastectomy and breast reconstruction after being diagnosed with the mutant gene.
Dr. Max Lehfeldt, a prominent Pasadena plastic surgeon, performed the reconstruction in February 2015 using Seri Surgical Scaffold, a silk mesh support for soft tissue. In Knecht’s case it was supposed to bolster the temporary, saline-filled implants that replaced the breast tissue removed during her surgery.
Immediately after the operation, Knecht experienced excruciating pain, she said in her lawsuit and medical board complaints.
She later learned — from other doctors who performed corrective surgeries — that the mesh had failed to adhere to her chest wall and was not supporting the implants, which were larger and heavier than promised, according to her lawsuit and the medical board complaints.
She also discovered that at the time of her surgery, the Seri Surgical Scaffold, although cleared by the Food and Drug Administration for other uses, was not approved for breast reconstruction.
Knecht’s lawsuit contended that Lehfeldt’s pushing of the device and his failure to inform her of its “off-label” use was driven by his financial relationship with its maker, Allergan, which federal records show had paid him more than $400,000 over three years to research and promote it.
Had she known of that relationship before her surgery, she would have made different choices, Knecht said.
“He appears to have a vested interest in this product, which seems to have caused a conflict of interest,” she said in her first medical board complaint, in August 2016, which also accused Lehfeldt of withholding information about postoperative complications.
“By not acknowledging this product’s failure in my case, Dr. Lehfeldt has caused severe harm and has demonstrated a flagrant disregard for the standard of care and ethics,” she said in the complaint, a copy of which she provided to The Times.
Knecht’s lawsuit alleged that Lehfeldt had conducted a clinical trial of the device for Allergan and used the same technique in her surgery, making her an unwitting participant in an “experimental” procedure. Three months after her operation, the FDA issued Allergan a warning letter, telling it to stop marketing the scaffold for breast reconstruction because it was an unapproved use.
Lehfeldt did not respond to The Times’ requests for an interview. Court records show he denied the allegations in Knecht’s lawsuit, including that the off-label use of the Seri mesh was inappropriate. He also said there was “no evidence presented” that her surgery was experimental.
Lehfeldt and his insurance company ultimately settled Knecht’s lawsuit for $1 million, without admitting liability. Allergan also settled with Knecht for an undisclosed sum sealed by a confidentiality agreement.
In December 2016, the medical board closed its investigation of Lehfeldt without pursuing disciplinary action. The vast majority of complaints are resolved that way, according to a Times investigation that found a pattern of leniency in the board’s discipline of doctors accused of harming patients.
“I was never interviewed,” Knecht said. “One of the biggest problems with the medical board is that it is so one-sided toward doctors.”
During discovery for her lawsuit, Knecht’s attorneys obtained records that Lehfeldt had submitted to the board in response to her complaint.
They included a 10-page “summary of treatment” in which he said that he had explained all options to Knecht before her surgery and that she ultimately chose to use the Seri Surgical Scaffold over another device. He also said he disclosed his research and training affiliations with Allergan to her.
“I advised [her] that my recommendations are driven purely on what is best for the patient and their specific clinical circumstances,” he told the board, denying that he concealed any of his concerns or findings from her.
“Everything was clearly explained in the operative report and to WK post operatively,” he wrote, using Knecht’s initials.
Knecht and her husband, Dr. Kalman Edelman, a gastroenterologist who was with her at the consultation, “emphatically dispute” those assertions and others outlined in the summary, according to a second complaint she filed with the medical board in March 2018.
That complaint also alleged that Lehfeldt had altered Knecht’s medical records by adding a notation to falsely indicate he had fully informed her about the Seri mesh and alternative treatments before her surgery. Knecht had copies of her original records and spotted the discrepancy.
During a 2017 deposition in the malpractice lawsuit, Lehfeldt acknowledged adding the notation to her chart — 21 months after her surgery — but said his failure to flag it as new information, as required, was merely an oversight.
“I added an additional clarification to one of the consult sheets, given the discussion we’d had, and I failed to initial and date it,” he said.
After Knecht’s second complaint, the board reopened its investigation and is now pursuing disciplinary action against Lehfeldt. He is accused of repeated negligent acts in his care of three patients, including Knecht, according to a second amended accusation filed in February.
In Knecht’s case, the board’s accusation alleges he altered her records without noting that his changes were made nearly two years after her surgery.
“His failure to sign and date the entry constitutes a departure from the standard of care,” the accusation reads.
A spokesman for the medical board declined to comment.
Medical device manufacturers and drugmakers must report their payments to doctors, which are then posted on the federal government’s Open Payments website.
But physicians are not required to inform their patients of those relationships, which can create conflicts of interest.
Knecht said she considered that to be a huge hole in patient safety and sought out her elected representative to remedy it. After meeting with her, Assemblyman Adrin Nazarian (D-North Hollywood) introduced legislation to mandate those disclosures.
The bill as written would have required doctors to disclose in writing the names of all drug and device companies they received payments from. The disclosures were to be made during patients’ initial office visits and then once a year thereafter, and doctors also would be required to provide a written notice to patients about the Open Payments website.
The California Medical Assn. opposed the bill, and in an email to members in May, the CMA’s political action committee sought donations to help kill it.
“This bill only adds to the litany of unnecessary burdens that divert physicians away from what they should be doing — providing care to their patients,” it said.
Patient advocates have long complained that their efforts to push legislators for meaningful change have been hamstrung by the politically potent doctors group, which has close ties to Gov. Gavin Newsom. A recent proposal to significantly increase medical license fees to beef up enforcement and another to shift the balance of the medical board from a physician majority to a public member majority were modified or cut from a pending Senate bill amid CMA lobbying.
Robert Fellmeth, executive director of the Center for Public Interest Law at the University of San Diego, which sponsored AB 1278, has called the CMA a “pernicious cartel” that consistently fights to starve the medical board of the funds it needs to investigate doctors.
Nazarian said the CMA opposition “was the main driving force” behind his decision to amend the bill so that it no longer requires doctors to tell their patients which companies, if any, are paying them. He said he chose to drop that provision because he feared trying to salvage it would doom the entire bill.
As it heads to a Senate Appropriations Committee hearing Aug. 16, the bill now only requires physicians to notify their patients of the existence of the Open Payments website and to post a notice about it in their offices.
Knecht said she is hopeful, but not optimistic, that the original provisions might be restored at the next step in the legislative process.
“The bad thing is the onus is still on the patient to find out about their doctors,” she said. “That’s kind of a tragedy.”
Distributed by Tribune Content Agency, LLC.