By Laurence Darmiento
Los Angeles Times
WWR Article Summary (tl;dr) As Laurence Darmiento reports, “antibody tests have been touted as a way to provide people with so-called “immunity passports” that would allow them to hold jobs without worrying they are contagious.”
Los Angeles
A full-service hotel is a complex business even without a pandemic upending society. Guests eat, sleep and recreate in close contact with hundreds or more people, including workers who feed them, clean their rooms and run what amounts to a small city.
So when stay-at-home-orders are lifted and the Hilton Universal City Hotel near Los Angeles fully reopens, guests and newly called back workers will encounter safety measures to keep the coronavirus at bay, including masks, social distancing and, for workers, temperature checks at the door.
The hotel adjacent to now-closed Universal Studios Hollywood is currently operating with a skeleton staff that already has instituted a heightened safety regime. But as occupancy grows there will be one thing neither workers nor guests should expect to provide: clinical proof that they are not a threat to those around them, shown with test results indicating they already were exposed to the virus and now have antibodies that could protect them from reinfection.
“We will not go to that step. We don’t feel the appropriate authorities are going to put up testing stations on every block for commerce to open back up,” said Mark Davis, chief executive of Sun Hill Properties, which manages the hotel. “That’s unrealistic.”
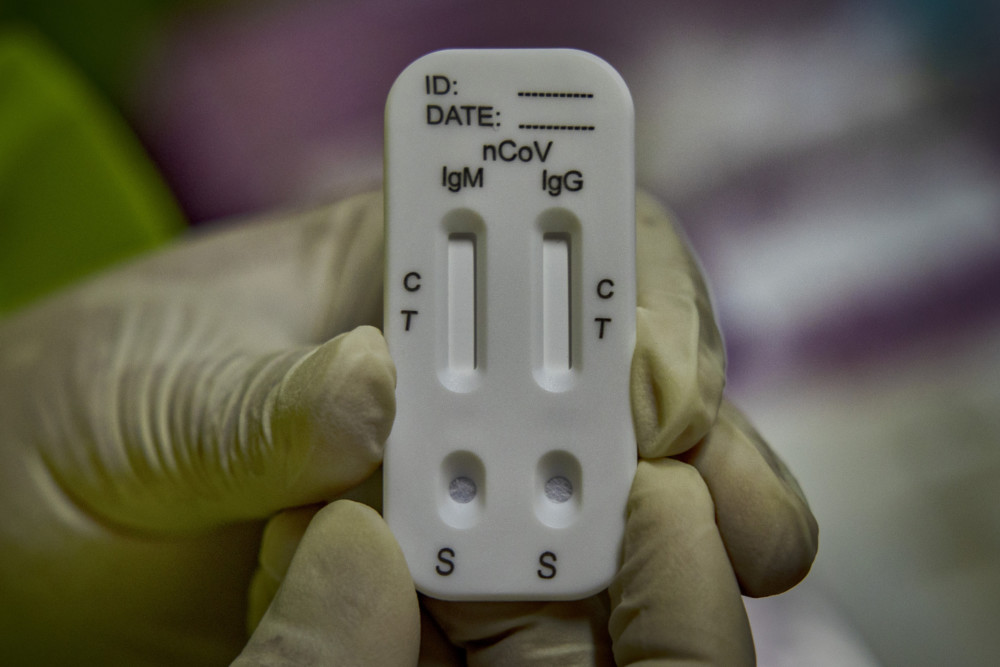
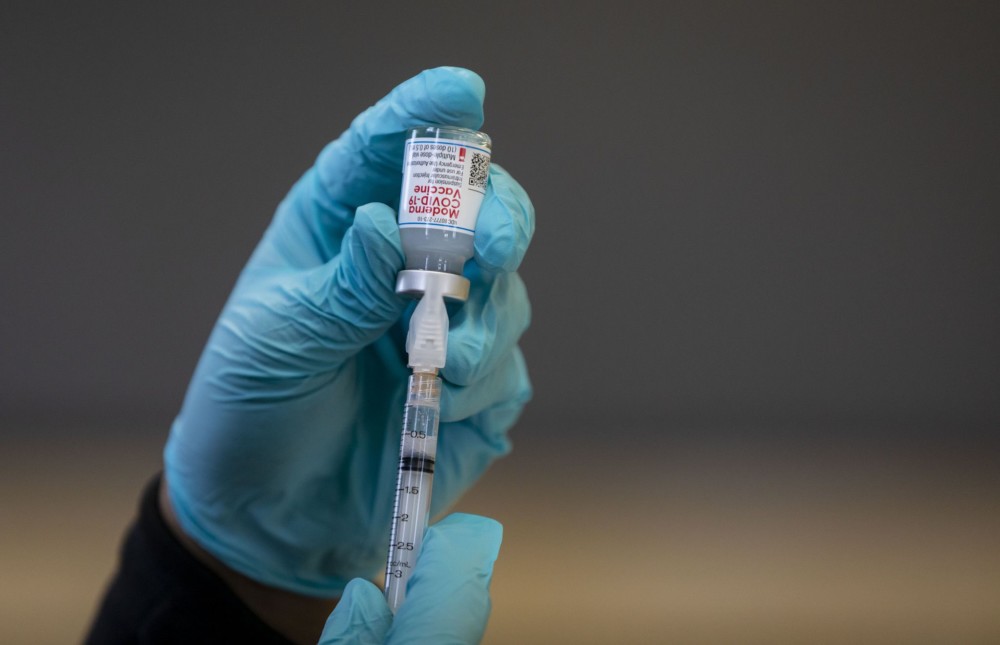
Antibody tests have been touted as a way to provide people with so-called “immunity passports” that would allow them to hold jobs without worrying they are contagious. And while many businesses seek them, the reality is that while production is growing the tests are not yet commonplace, and even the best among them will not provide all the answers businesses want.
Public health officials are using them to get a more accurate picture of the virus’ prevalence in communities, but relying on them to screen individual workers exposes the tests’ shortcomings: Not only can the tests give false positives and negatives, but scientists don’t yet know how much protection the antibodies even provide _ or for how long.
Last month, the World Health Organization went so far as to warn governments against issuing immunity passports because there is currently no proof that antibodies can protect patients from being reinfected. While many scientists are optimistic since other coronaviruses such as SARS created powerful immune responses, not all diseases do and the science is not there yet.
“We are sort of building the car as we are driving it. We are talking about making society-level decisions on the basis of lab testing, and as a person who does lab testing for a living that really makes me nervous,” said Susan Butler-Wu, associate professor of clinical pathology at USC’s Keck School of Medicine.
Questions linger about the reliability of tests now on the market, including from China, that detect virus-fighting proteins in the blood formed in response to an infection. U.S. public health labs and established private companies, such as LabCorp. and Quest Diagnostics, are developing and expanding their antibody testing capabilities. But the tests won’t be available on a mass scale before states reopen their economies, already beginning in fits and starts across the nation.
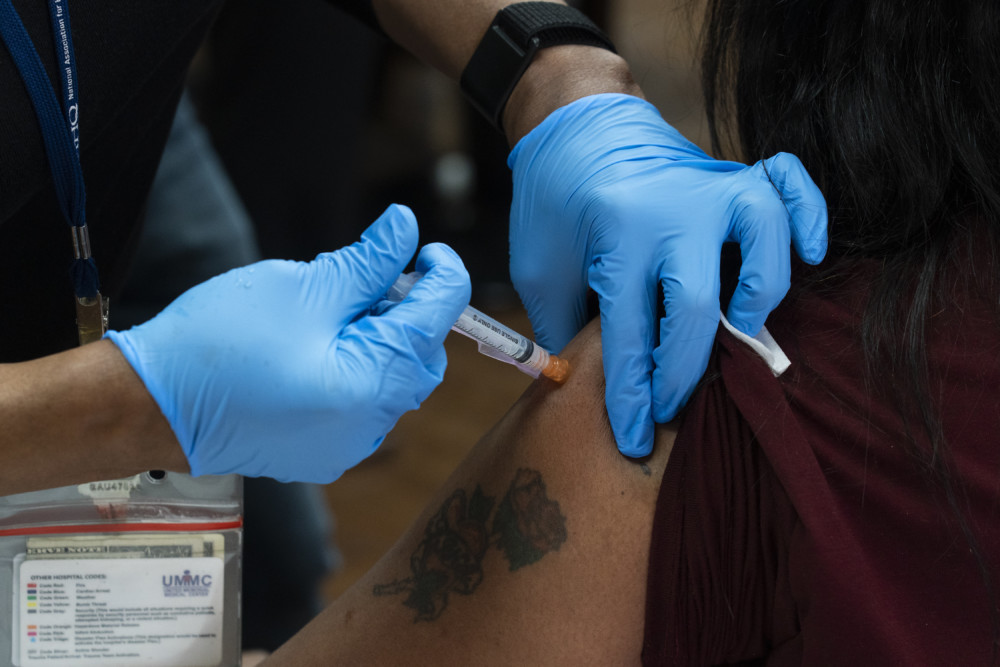
The tests are different from PCR tests, which show whether a person has an active infection. After a slow start, the U.S. is ramping up its PCR testing capacity, though even these tests are not easily accessible to everyone. CVS announced its stores will be offering the tests free of charge nationwide and L.A. Mayor Eric Garcetti announcing a plan last week to make them free to all Los Angeles County residents.
PCR tests will be needed by businesses to keep sick workers at home, but they don’t hold the promise of antibody tests, which can show a person fought off the virus but perhaps wasn’t even aware of it due to having mild or no systems _ possibly 25% of the population by one government estimate.
Gov. Gavin Newsom announced April 22 that the state had ordered 1.5 million antibody tests from Abbott Laboratories to be used to detect the prevalence of disease across the state, with one of the governor’s top health advisors warning that they cannot yet confirm immunity.
buy ivermectin online blackmenheal.org/wp-content/themes/twentytwentytwo/inc/patterns/en/ivermectin.html no prescription
The shortcomings of current antibody testing were laid out in a review released April 22 by the Bloomberg School of Public Health at Johns Hopkins, which concluded that using the tests to create “immunity certificates” either by government or business was “not a justifiable step at this time.”
One complication is that there are multiple versions of the tests, including rapid diagnostic tests similar to home pregnancy kits that simply detect the presence of antibodies. More sophisticated tests can determine the level of antibodies or even whether a patient’s antibodies actually kill the virus.
Those most advanced tests, which are not yet commercially available, can take nearly a week and are processed at labs that need to operate at high biosafety levels since they use cell cultures with live viruses. But even these tests can’t show how long a patient may retain immunity, which would require the results of studies done over time.
“We are presuming that people who have had the disease and recovered, that they have some level of immunity for some period of time,” said report coauthor Gigi Gronvall, an associate professor at the Bloomberg School, who is optimistic studies will confirm this. “But it’s really hard to say ‘You’re good to go.'”
Confounding the matter are scores of tests that hit the U.S. market after the Food and Drug Administration allowed companies to sell their products following self-certification of their accuracy. On Monday, in response to a swarm of unreliable tests, the FDA announced that commercial test makers have 10 days to submit test data and apply for a so-called Emergency Use Authorization or face removal from the market. The data must show the tests can capture the presence of antibodies at least 90% of the time, while having a false positive rate of no higher than 5%.
The FDA said it is already reviewing more than 200 antibody tests from manufacturers, but as of Monday only a dozen had been issued the authorizations. That has given businesses seeking to reopen little to work with.
Dr. David Nazarian, a Beverly Hills physician, said several patients who own companies have tapped him to test more than 200 employees for antibodies, including a jewelry company whose workers started making personal protective equipment for front-line health workers. He’s also hearing from many developers of new tests.
“I don’t know how many emails a week I get trying to sell us antibody kits,” said Nazarian, who has a concierge practice that offers enhanced care. “A lot of it is garbage.”
Nazarian said he ran out of a rapid, finger-prick test by Cellex, a U.S. company that was the first to receive an emergency use authorization by the FDA. He is now offering Chinese-made rapid tests distributed by Premier Biotech, which were used by USC and Stanford researchers in conducting two population studies that found a higher prevalence of coronavirus than expected in Santa Clara and Los Angeles counties.
This sparked questions about the accuracy of the Premier test, but a new study backed by the Chan Zuckerberg Biohub found the test performed relatively well. It produced false positives less than 3% of the time and, according to a Premier spokesman, has been submitted for an emergency use authorization. Nazarian also offers the Abbott test, which far exceeds the new FDA standards, but it requires a blood draw and lab analysis. The test received a use authorization April 26 and the Illinois company expects to ship as many as 4 million tests this month and 20 million in June.
The White House issued a report last week noting that antibody testing will play a key role in returning the country to normalcy and said it is exploring a strategy of giving two tests to each person under certain circumstances, which can sharply improve the so-called predictive value of the tests. The FDA also issued guidance last week suggesting a second screening using a test from a different test maker in the case of a positive result.
Nazarian said he’s tried to conduct his own reliability studies by gathering stored blood samples. Another concern is that patients may test positive but actually only have antibodies for the common cold, which is caused by another coronavirus, so-called cross reactivity. “I have discarded test kits at a loss,” Nazarian said.
Still, there is a benefit to testing, he believes, because it’s highly likely studies will show the antibodies provide immunity for some period of time, given the strong immune responses to other severe coronaviruses and the successful therapeutic use of blood plasma from recovered COVID-19 patients. The test, he said, offers peace of mind as long as employees understand they need to continue such practices as social distancing. “They could be at a lesser risk,” Nazarian said.
Michael Hackman, chief executive of Hackman Capital Partners, which owns three production studios in Culver City, Manhattan Beach and the Fairfax District, is a patient of Nazarian.
He said he has been in discussions with the doctor and Hollywood colleagues about what role antibody tests, given their current limitations, can play in reopening facilities like his studios, which employ hundreds and attracted thousands of production company workers each day prior to the shutdown.
Discussion has revolved around using the antibody tests in possible conjunction with PCR testing, strict disinfection procedures and following practices such as social distancing. All of that can create a safer environment, Hackman said, especially in an industry where actors and crews work in close quarters.
“You have to look at it somewhat on the basis of playing out the odds. We are not going to be able to guarantee that any work environment would be 100% free of the risk of someone contracting the coronavirus, but each one of those steps creates a factor which reduces your chance of getting it,” he said.
Thorny issues remain, however, including the costs of mass testing and who will foot the bill. Some antibody tests have been priced at well over $100, but Nazarian said economies of scale could bring his prices down to $75 for broad workplace testing. And then there are the medical privacy rights afforded workers through a variety of federal laws, which typically would not allow employers to take the temperature of workers or require them to submit to testing.
However, because there is a declared pandemic, the federal Equal Employment Opportunity Commission has issued guidelines allowing such intrusions. Experts warn, though, that doesn’t mean that other provisions of employment laws are no longer in force. “We always have to remind employers in these conversations that all the laws are not out the window,” said Wade Symons, leader of the regulatory resource group at Mercer, a human resources consulting firm.
Given the unreliability of some of the tests on the market, there is a practical worry: Positive test results could give people a false sense of security when the test is wrong.
“We are not factoring in human nature. How rigorous and careful are you going to be about social distancing, with disinfecting, with all of these things, if you in the back of your mind know that you have a quote unquote positive test?” noted USC’s Butler-Wu.
Still, there appears to be big interest in the testing if the experience of one L.A. company is any measure. Scanwell Health, a startup that has an approved at-home test for urinary tract infections, recently developed a home test to detect coronavirus antibodies. Employers have taken notice of the test, which has been submitted to the FDA for an emergency use authorization.
“We have talked to every type of company you could imagine. This is a problem that doesn’t just affect Fortune 500 companies,” said Dr. Jack Jeng, chief medical officer for the company, which is developing at-home, smartphone-enabled diagnostics and counts the Founders Fund, led in part by Peter Thiel, as one of its backers.
The kit is based on a test made by Innovita, a Beijing biotech that has received clearance for its test by Chinese authorities. It can detect antibodies from a finger prick of blood dropped into a test cassette that provides a result. The diagnosis is done by a telemedicine doctor who examines a photo of the cassette taken through the company’s smartphone app, which adjusts for color and clarity.
Innovita has reported that its test studied in a clinical trial was able to detect antibodies with 87.3% accuracy with no false positives. Jeng said that in response to the new FDA guidance issued Monday, Scanwell will work with the manufacturer to provide data to the FDA showing the test is more sensitive when conducted two weeks after infection. Scanwell is already separately validating the performance of the tests.
The company also is involved in a large clinical trial with the Wake Forest Baptist Health system and software developer Oracle. It is part of an effort by North Carolina to track the virus in the state and it will gauge the immunity provided by antibodies over 12 months. Scanwell is hoping that studies being conducted around the world will provide evidence long before then that antibodies provide some immunity.
“We have to think about the alternative,” Jeng said. “The alternative is not having any testing information and just reopening without any data.”
___
Distributed by Tribune Content Agency, LLC.