By Emily Kopp
CQ-Roll Call
WWR Article Summary (tl;dr) The new test allows a person to take the test at home and then send it to a lab for results.
WASHINGTON
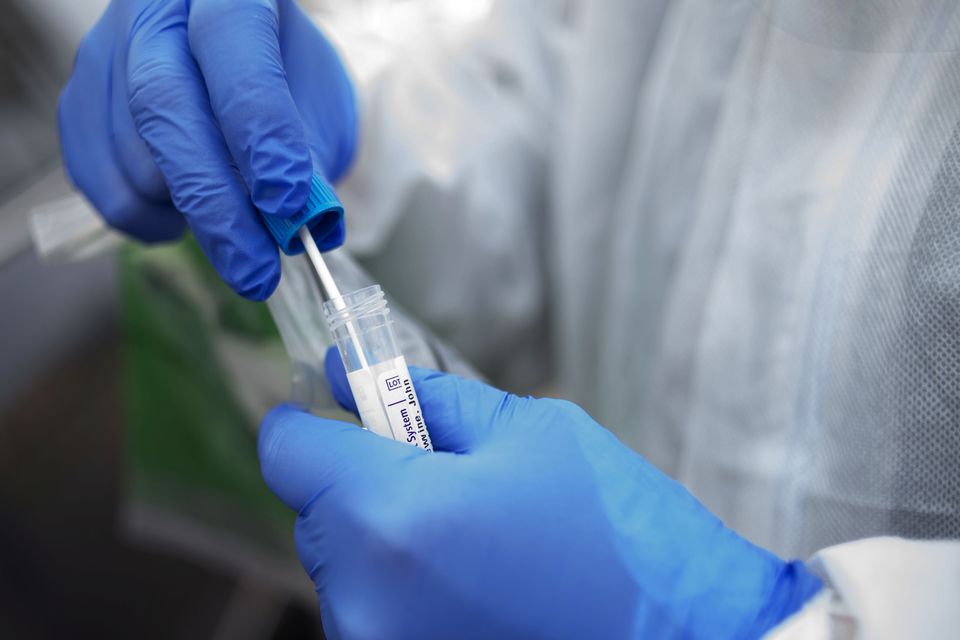
The Food and Drug Administration announced Tuesday that it had authorized an at-home test for COVID-19 that patients can administer themselves.
The test, manufactured by Laboratory Corporation of America, or LabCorp, allows for patients to collect a sample from their noses and ship it into one of the corporation’s labs for testing.
A physician or another health care provider would need to order the test, according to a letter from FDA to LabCorp.
On April 5, LabCorp requested the FDA expand its March authorization to test for the coronavirus that causes COVID-19 to allow for at-home specimen collection.
FDA “worked with LabCorp to ensure the data demonstrated from at-home patient sample collection is as safe and accurate as sample collection at a doctor’s office, hospital or other testing site,” Commissioner Stephen Hahn said in a news release.
The test includes nasal swabs, saline and insulated packaging, the agency said.
Early at-home tests were plagued by questions about accuracy.
FDA changed its guidance in order to approve the collection of samples from the front of the nose rather than the depth of the nasal cavity for symptomatic patients in late March.
Self-swab tests can help conserve the personal protective equipment and health care personnel required for tests administered at the point of care such as a hospital or doctor’s office, according to the Federal Emergency Management Agency.
A national shortage of medical swabs remains a barrier to readily available testing. The FDA said Tuesday it is examining whether more widely available cotton swabs, like Q-tips, can be used to collect samples.
But there are concerns with “cross reactivity due to inherent genetic material in cotton swabs,” the FDA said.
In other words, the cotton material in a standard Q-tip could falsely trigger the test for the coronavirus, while the cotton material in LabCorp’s swabs have been tested to ensure they don’t, according to FDA spokesperson Emma Spaulding.
Health care providers and emergency first responders who were exposed to the coronavirus will be first in line for the tests, the company said.
That could get people back to work after suspected exposure to the virus without a 14-day quarantine.
Right now, guidance from the Centers for Disease Control and Prevention states that health care providers and other so-called “critical infrastructure” workers can be required to report back to work immediately after exposure if they wear a mask.
Unions like National Nurses United and some epidemiologists have raised concerns that without tests, that policy could fuel clusters of infections at hospitals, warehouses and grocery stores.
The fact that the test is being prioritized for health care providers shows there is a long way to go before testing is readily available to all and the United States can safely resume economic activity, said Michael Carome, director of the health research group at Public Citizen, who has raised concerns about a shortage of tests for weeks.
“The lack of testing in this country has been the foundational problem and the biggest failure of our government to respond to this pandemic,” Carome said.
FDA said the test will be available in “most states” and “in the coming weeks.” FDA referred questions about which states will receive the first shipments of tests, and when they will begin receiving them, to LabCorp.
LabCorp did not immediately respond to questions.
Hahn and other Trump administration officials have previously given optimistic and ultimately inaccurate timelines for the ramp up of testing across the United States.
___
Distributed by Tribune Content Agency, LLC.