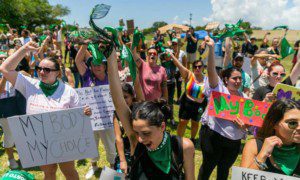OPINION
By Ellen Laan and Leonore Tiefer
Los Angeles Times.
Many women report losing their desire for sex, some temporarily, some permanently. Is this a relationship problem, a normal aspect of life changes or, as the pharmaceutical industry maintains, an “unmet medical need”? That was the question under consideration for two days of meetings recently, during which the Food and Drug Administration heard from sexual medicine experts and women with sexual complaints.
It was a rowdy meeting by sedate, scientific standards, and months of public relations campaigning had preceded it. The International Society for the Study of Women’s Sexual Health, which is largely funded by the pharmaceutical industry, had joined with Sprout Pharmaceuticals and other companies with skin in the game to develop two slick campaigns, “eventhescore.org” and “womendeserve.org,” which argued that the FDA’s failure to approve a drug to treat women’s sexual problems was “sexist.” After all, men have Viagra and its various relatives.
The patients told stories of their frustrations and distress, but they appeared to have been coached to demand drug solutions.
They acknowledged that their travel expenses to the meeting had been paid. Wearing matching green scarves and buttons proclaiming “#WomenDeserve,” the women described the mixed results and side effects of their various off-label treatments, including implanted testosterone pellets, testosterone gels and antidepressants. They insisted they had no nonmedical problems. Their desire had simply “turned off like a light switch,” as one woman said, sometimes as much as 30 years earlier, and they wanted it back, routine and predictable.
As professional sexologists and advocates of women’s sexual rights, we were horrified by the campaigns’ use and abuse of the language of equality to pressure the FDA to approve a potential billion-dollar blockbuster “pink Viagra.” The only two drugs for women’s sexual dysfunctions that have come to the FDA in the 16 years since Viagra was approved were rejected. One was Sprout’s drug, flibanserin, then owned by a German company. The drugs for women didn’t work and were unsafe. That’s not sexism, that’s proper regulation.
The campaigns to “even the score” are deceptive for several reasons.
First, they repeat the statistic that 43% of American women have a sexual dysfunction. That number is from a 1994 survey that asked women whether they had any kind of sexual problem (yes or no) without asking whether the problem bothered them. The senior author of the study has said for years that this statistic is being misused. Independent research has found that about 10% of women have distressing sexual concerns, most notably pain or a low desire for sex. Furthermore, many of these concerns can be remedied without drugs or medical procedures. But the 43% figure creates a sense of urgency for a supposed condition that deserves to be treated by a pill.
Second, the campaigns claim that the FDA has approved 26 drugs for male sexual dysfunction and zero for women. This is nonsense. The 26 include different versions of similar drugs, and mostly feature testosterone, which an FDA advisory committee recently warned is wildly oversold to men for age-related normal changes, has serious risks and has not been approved for sexual dysfunction. Yet you will see “26-0” repeated in the media.
buy silvitra generic buy silvitra online no prescription
Third, there is absolutely no evidence for womendeserve.org’s claim that “a biological lack of desire to have sex negatively impacts 1 in 10 American women.” No diagnostic test has identified any biological cause, brain, hormone, genital blood flow, for most women’s sexual problems.
On the contrary, abundant evidence shows that low sexual desire in women typically reflects a difference in desire between two partners. It is unethical and unscientific to attribute a couple’s discrepancy in desire to the woman’s biological deficit. In study after study, women’s response to both test medications and placebo drugs is high. These repeated findings do not support the “unmet medical need” theory.
Indeed, the scientific community regards most sexual problems in healthy people as related to what is going on in the bedroom, the relationship, the partners’ individual lives and shifts in cultural norms. The incentives for sex, or for avoiding sex, are far more important in understanding a couple’s issues than one partner’s biology.
If the pharmaceutical industry were truly concerned with women’s sexual well-being, companies would market drugs that are effective for women whose sexual problems are caused by physical problems or disease, such as diabetes, multiple sclerosis and spinal cord injuries. Yet efforts to test drugs for narrow markets have been curtailed on several occasions as the industry pursued its blockbuster dreams.
The partnership between sexologists and drug companies is full of conflicts of interest and has sullied our field. Fortunately, judging by their comments at the recent meeting, we are hopeful that the FDA experts are not being taken in by ill-advised initiatives. We hope that the public won’t be, either.
(This article appeared as an opinion piece in the L.A. Times. Ellen Laan is an associate professor of sexology at the University of Amsterdam, the Netherlands, and a Kinsey Institute research fellow. Leonore Tiefer is a clinical associate professor of psychiatry at NYU School of Medicine and founder of the New View Campaign, newviewcampaign.org.)